The RCP works in partnership, nationally and internationally, to develop physicians throughout their careers.
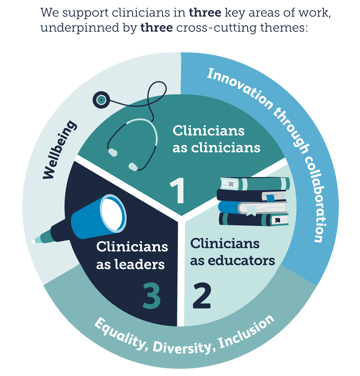
We develop clinicians in three key areas:
Across these programmes, our portfolio is underpinned by three cross-cutting themes: equality, diversity, inclusion; innovation through collaboration; and wellbeing.
Our portfolio includes a wide-ranging combination of activities, using diverse professional expertise: educationalists, clinical faculty, assessment specialists, learning technologists, curriculum and business development expertise, and a business support and marketing team to underpin developments and operational delivery.
We also offer our library service; podcasts; free e-learning for RCP members and deliver bespoke, commissioned workshops, leadership programmes and more.
Looking to develop your career?
All our courses in one place.
Filters
Becoming a physician in the UK
To become a physician in the UK, most people will follow the pathway of:
- Medical school (5 years)
- Foundation training (2 years)
- Internal medicine training (2-3 years)
- Higher specialty training (4+ years), leading to certificate of completion of training (CCT)
There are many organisations, including The Federation of the Royal Colleges of Physicians, that are responsible for planning and delivering this physician training pathway in the UK. The RCP has developed an infographic setting out the responsibilities of these various organisations.
Alternatively, doctors who have not completed a GMC-approved training programme but have the knowledge, skills and experience equivalent to the approved curriculum may still join the specialist register through the portfolio pathway (formerly known as the certificate of eligibility for specialist registration, or the CESR route).
Interested in a podcast?
The RCP Medicine podcast has been developed to provide you with learning that you can listen to on-the-go.
Listen now
Learning support for physicians at a time and place to suit you
Whether at home, online or at our beautiful library in our London building by Regent's Park, we support physicians to further develop their careers.

RCP onlineEd
Our online learning platform for e-learning wherever you are.
It's easy to use, and our online courses are free for RCP members.
Access RCP OnlineEd
RCP library
Our library supports fellows, members and RCP students to meet their professional development and educational goals.
RCP library
Commission us
The RCP designs and delivers high quality education for many partners in the UK and across the world.
Commissioned work









